Research progress and industrialization direction of zinc iron flow batteries
Classification:Company News
- Author:ZH Energy
- Release time:May-09-2024
【 Summary 】By utilizing the high activity and multi electron chemical reaction characteristics corresponding to zinc metal, zinc based flow batteries (ZFBs) can be obtained with advantages such as low cost, high
Due to the sharp reduction in non renewable fossil energy reserves, developing renewable clean energy and optimizing the existing energy structure has become a universal consensus in the international community. In this context, solar energy, wind energy, and other industries are becoming increasingly mature and rapidly expanding in scale, and have become important pillars of China's energy transformation. However, due to factors such as wind speed, solar radiation intensity, and time, wind and solar energy can generate unstable, intermittent, and other non-stationary power outputs during the power generation process. Especially when used on a large scale, it is easy to have an impact on the power grid, posing huge risks to the quality of electricity provided by the power grid and safe operation, and facing serious problems of grid connection and consumption. Therefore, developing supporting renewable energy storage systems, achieving stable power output, and improving power quality are important measures to ensure the safety of grid connection.
Liquid flow batteries are one of the highly anticipated options. In previous articles, we have introduced the relevant progress research of all vanadium, iron chromium, and all iron liquid flow batteries. This article will focus on zinc iron liquid flow batteries. By utilizing the high activity and multi electron chemical reaction characteristics corresponding to zinc metal, zinc based flow batteries (ZFBs) can be obtained with advantages such as low cost, high safety, flexible structure, and high energy efficiency. Among them, zinc iron flow batteries have greater cost advantages due to their high abundance of iron.
Common zinc iron flow batteries have two systems: acidic and alkaline: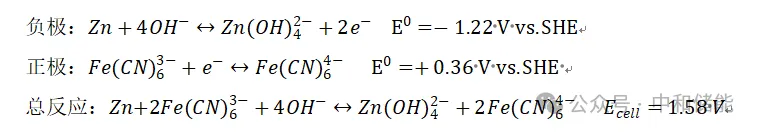
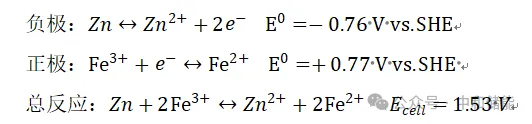
The research on ion exchange membranes is a key direction for zinc iron flow batteries. Yuan et al. developed a low-cost polybenzimidazole film for alkaline zinc iron flow batteries, which has high mechanical strength and chemical stability and can provide high ion conductivity. In addition, the battery also uses three-dimensional porous carbon felt as a guiding material for zinc deposition and dissolution, effectively suppressing the formation of zinc dendrites. Through these improvements, the power density, energy efficiency, and stability of alkaline zinc iron flow batteries have been significantly improved. In addition, the practicality of this type of battery has been confirmed, with the overall cost of the battery pack being less than $90/kW h [2]. Chang et al. developed a mixed matrix porous polyolefin based membrane (DM-HM) filled with functionalized hollow spheres and applied it for the first time in alkaline zinc iron flow batteries. Due to the high alkali resistance and porous structure of hollow nickel silicate spheres, the prepared mixed matrix DM-HM has good structural stability and ion conductivity, maintains low membrane resistance, and exhibits good efficiency at high current density, providing new ideas for the commercial application of alkaline zinc iron flow batteries [3]. In addition, Yuan et al. also designed a negatively charged nanoporous membrane with pore walls and surfaces. Due to the mutual repulsion between the negatively charged Zn (OH) 42- and the negatively charged porous membrane, zinc ions will deposit on the three-dimensional porous carbon electrode in the opposite direction of the membrane. Using this negatively charged nanoporous membrane, zinc dendrites are still not generated after 240 cycles of operation under conditions of 80-160 mA/cm2[4]。
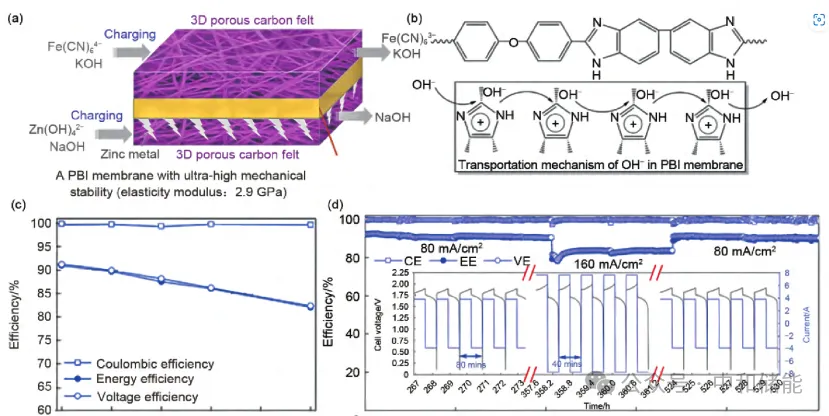
Recurrent process cyclic process[5]。
In addition, in zinc iron flow batteries, zinc metal undergoes processes such as corrosion, passivation, hydrogen evolution reaction (HER), deformation, and dendrites during the electrochemical process, and their interactions are enhanced, thereby affecting the practical application of flow batteries. It is necessary to suppress these processes. Specifically, the formation of dendrites will lead to an increase in the surface area of the negative electrode, thereby accelerating the precipitation of hydrogen gas and causing changes in the pH value of the local electrolyte on the surface of the electrode. The generated OH - will continue to participate in the reaction and form electrochemical inert byproducts, which will deposit on the negative electrode surface. The ZnO passivation film will further cause an increase in the unevenness and polarization of the electrode surface, thereby promoting the formation of dendrites. Therefore, research on zinc negative electrodes is also one of the key focuses of zinc iron flow batteries.
The most common method for inhibiting zinc dendrites is to add inhibitors to the electrolyte, and the inhibitory effect on zinc dendrites varies depending on the type of inhibitor added. To add metal ions to the electrolyte, it is necessary to have a high resolution hydrogen potential ion with a deposition potential lower than that of zinc, ensuring that it becomes a substrate electroplating layer before zinc deposition, in order to improve the uniformity of zinc deposition on the electrode and suppress the formation of zinc dendrites. Banik et al. found that adding PEI (polyethylene imine) to the electrolyte can significantly change the tip of zinc dendrites into smaller spherical dendrites without causing severe negative electrode polarization, thereby reducing the threat of zinc dendrites to ion exchange membranes [6]. Through research, Beshore et al. found that when gel was added to the electrolyte, the uniformity and compactness of zinc deposition on the electrode were greatly improved, and the volume of zinc dendrites was also reduced. However, the resistance of the flow cell would also increase due to the reduction of electrolyte fluidity [7].
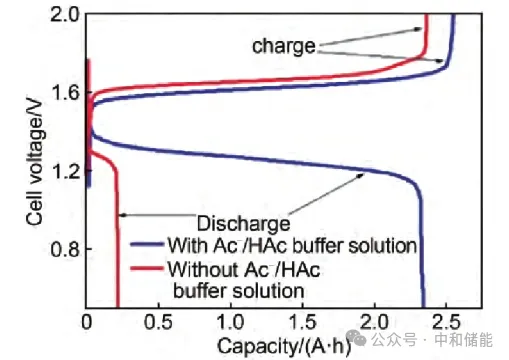
In addition, there have been studies that have optimized and innovated battery structures. A study has combined acidic zinc iron flow batteries with alkaline zinc iron flow batteries to design a new type of acid-base mixed zinc iron flow battery. Due to the good solubility and electrochemical activity of zinc salts in the negative electrode electrolyte of alkaline zinc iron flow batteries; In the positive electrode electrolyte of acidic zinc iron flow batteries, Fe2+/Fe3+has good solubility and electrochemical activity. At the same time, these two electrolytes require rich raw material content and low cost. Under this idea, the single separator battery structure was changed to a double separator structure, and another neutral electrolyte chamber was added as a buffer solution. The power density of the battery can reach 676 m W/cm2, and the investment cost is less than 100 USD/(k W · h), far below the 2023 target set by the US Department of Energy [150 USD/(k W · h)] [8].
产品系列:
液流电池-电极/隔膜
LAB系列研发示范装置
储能系统度电成本计算器NeLCOS®
大唐中宁启动200MW/800MWh大容量长时共享储能项目招标
全钒液流储能进入GWh时代!
融中财经访谈:中和储能谢伟,打造高技术壁垒液流电池材料产品

