Water based redox flow batteries based on neutral alkali metal iron/ferrous cyanide and polysulfide electrolytes
Classification:Industrial News
- Author:ZH Energy
- Release time:Jun-18-2024
【 Summary 】Polysulfide redox pairs have been proven to be a promising candidate for redox reactions, widely used in water-soluble polysulfide bromide (PSB) systems and recently in non-aqueous lithium sulfur flow
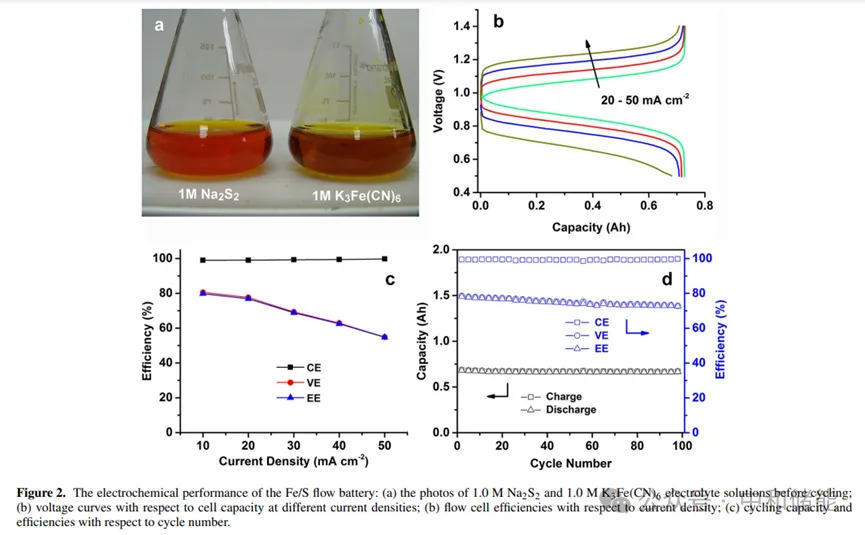
Research Highlights Polysulfide redox pairs have been proven to be a promising candidate for redox reactions, widely used in water-soluble polysulfide bromide (PSB) systems and recently in non-aqueous lithium sulfur flow chemistry. Iron/ferrous cyanide pairs are commonly used as electrochemical standard redox pairs due to their clear redox behavior and fast electron transfer kinetics. The paper presents a novel iron/ferrous cyanide polysulfide (Fe/S) redox flow battery, which uses alkali metal iron/ferrous cyanide positive electrode and alkali metal polysulfide negative electrode as active redox pairs with high solubility, low corrosiveness, and relatively harmless to the environment. Cobalt nanoparticles modified graphite felt is used as a catalyst on the polysulfide side. Material preparation The positive electrode electrolyte uses a solution of 0.05 M K æ Fe (CN) 6 in 1.0 M NaCl, with glassy carbon (GC) as the working electrode; The negative electrode electrolyte uses a solution of 0.05 M Na ₂ S ₂ in 1.0 M NaCl, with a catalytic cobalt nanoparticle modified glassy carbon (Co GC) electrode as the working electrode. research contents The author first observed the CV of the positive and negative redox electrolytes and found that iron/ferrocyanide redox exhibited good reversibility, with clear redox peaks at+0.32 V and+0.24 V (Figure 1a). Comparing the CV curves before and after Co electrodeposition, it can be found that Co nanoparticles indeed greatly enhance the electrochemical reactivity of polysulfides, and the peak current is much higher. In addition, in the CV curve of Co GC, there are multiple electron transfers within the cycling range, indicating that polysulfides may undergo more complex electrochemical reactions and may involve more polysulfide substances (Figure 1c). Subsequently, the author investigated the electrochemical performance of Fe/S flow batteries. The author used excessive polysulfide electrolytes in Fe/S flow batteries, limiting the negative electrode reaction to between S ₂ ² − and S4 ² −. The study found that the voltage curves of Fe/S flow batteries at different current densities clearly showed only one plateau, indicating that electrochemical transformation mainly occurred between S ₂ ² − and S4 ² −, without the observation of hydrogen evolution (Figure 2b). Furthermore, it was found that Fe/S flow batteries exhibit extremely high Coulombic efficiency (CE,>99%) in the current density range of 10 to 50 mA cm − ², indicating that the cross and self discharge reactions of redox materials can be ignored. However, the rapid increase in overpotential with current density indicates that the polarization of the battery is quite high. The voltage efficiency (VE) decreased from 80% at 10 mA cm − ² to 55% at 50 mA cm − ², and due to high CE, the energy efficiency (EE) followed the trend of VE (Figure 2c). This is because the ion conductivity of neutral electrolyte flow systems is low, and the transmembrane transport speed of non proton charge carriers is slow, usually with relatively poor rate performance. However, the results in Figure 2d indicate that Fe/S flow batteries exhibit good cycling stability, achieving relatively stable battery efficiency in 100 repeated charging/discharging cycles, with CE of 99%, VE of~75%, and EE of~74%. Fe/S flow batteries maintain almost constant cycling capacity, with an average capacity loss of 0.02% per cycle. The author further analyzes the impedance distribution in flow batteries to determine the key factors affecting performance in Fe/S flow batteries. The author placed a carbon cloth probe (Figure 3a) in the middle of the Nafion laminated film, impregnated Nafion resin into the carbon cloth to ensure good contact with the Nafion film and minimize the probe Nafion interface resistance. Using the equivalent circuit model of constant phase element (CPE) (Figure 3b, illustration), numerical fitting was performed on EIS. It was found that under fully charged and fully discharged battery conditions, the Ohmic resistance and charge transfer resistance of the entire battery and two half cells remained almost unchanged. The Ohmic resistance of the negative half cell (0.084 Ω, accounting for 39% of the entire battery) was lower than that of the positive half cell (0.132 Ω, accounting for 61% of the entire battery). In addition, the charge transfer resistance of the negative half cell (0.066 Ω) is lower than that of the positive half cell (0.079 Ω). Therefore, it can be considered that the battery impedance is more distributed on the positive side, which appears to be the rate limiting half cell in Fe/S flow batteries. In addition, by estimating the cost, the bulk prices of potassium ferrocyanide and sodium polysulfide are approximately $2 and $0.7 per kilogram, respectively, resulting in a total raw material cost of approximately $34 per kilowatt hour for Fe/S flow batteries with an oxidation-reduction material concentration of 1 M. According to recent cost analysis models, this is only one-third of the vanadium material cost for the VRB sulfate system (approximately $110 per kilowatt hour). In addition, the redox potential of iron/ferrous cyanide redox pairs is relatively low, and the durability requirements for battery module materials are lower than those of strong redox materials such as all vanadium, hydrogen bromide, zinc cerium, etc; For example, inexpensive battery component materials and hydrocarbon based ion exchange membranes are expected to be suitable for Fe/S systems, resulting in greater cost-effectiveness. Therefore, it is expected that the capital cost of Fe/S flow batteries will be relatively low, making them a promising low-cost energy storage technology. Although high current operation may be limited due to low electrolyte conductivity, considering its cost-effectiveness, Fe/S systems are still technically and economically feasible in energy oriented grid applications.
The Fe/S liquid flow system adopts a relatively environmentally friendly neutral electrolyte, avoiding irritating battery media such as strong oxidants, corrosive acids, or bromine. Fe/S flow batteries exhibit excellent electrochemical performance, with high battery efficiency (99% Coulombic efficiency and~74% energy efficiency) and good cycling stability, enabling long-term charging/discharging operations. Further research using carbon cloth probes revealed that the positive half cell appears to be the performance limiting side in Fe/S flow batteries. Due to the use of two types of redox materials and some battery materials that are very cheap and abundant, rather than based on redox active metals (such as vanadium based flow batteries, which are usually limited by resources and costs), Fe/S flow batteries can reduce costs and become an economically efficient candidate for energy storage technology.
The assembly method of Fe/S redox flow batteries is as follows: from one end to the center, each half cell is composed of stainless steel end plates, graphite plates, GFD graphite felt, and a Nafion film (N117) in the middle. The effective area of electrodes and membranes is 5 cm x 2 cm. Soak N117 in deionized water at room temperature for 24 hours before battery assembly. The GFD electrode used as the positive electrode is heat treated in air at a temperature of 400 ° C for 6 hours to increase its hydrophilicity. The negative electrode is a GFD (Co GFD) electrode modified with cobalt nanoparticles, with a Co loading of 47 mg cm − ². The positive electrode electrolyte uses 40 mL of 1.0 M potassium ferrocyanide aqueous solution, and the negative electrode electrolyte uses 60 mL of 1.0 M sodium disulfide (Na ₂ S ₂) aqueous solution. Use a peristaltic pump to pump the electrolyte from the electrolyte storage tank to the flow battery compartment at a flow rate of 20 mL min-1. Evaluate the charging/discharging cycle performance of flowing batteries using a constant current mode on a battery tester within a voltage window of 0.5-1.4 V.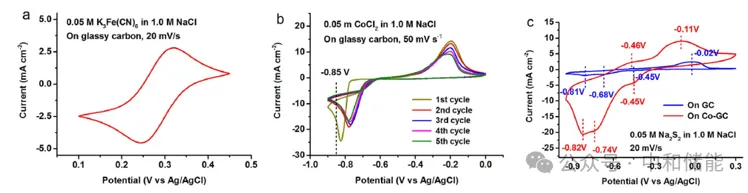
research conclusion
This article presents a novel Fe/S flow battery system that uses iron/ferrous cyanide redox pairs as the positive electrode electrolyte and polysulfide redox pairs as the negative electrode electrolyte. Fe/S flow batteries have outstanding electrochemical performance, with a CE of 99% and an EE of approximately 74%. Moreover, the flow battery exhibits excellent cycling stability, with a capacity retention rate of 98% in 100 cycles. Further research confirms that the positive half cell has been identified as the performance limiting side, as its ohmic resistance and charge transfer resistance are higher than those of the negative half cell.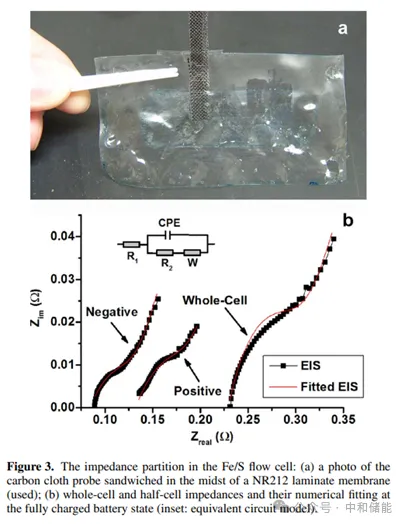
The main challenges currently faced by Fe/S flow batteries are: firstly, low power density, which may be caused by slow electrode reaction kinetics and high neutral electrolyte resistivity. These problems can be solved by using catalysts with higher catalytic activity and adding supporting electrolytes (such as KCl) to improve electrolyte conductivity. Another challenge is that the recyclability of Fe/S flow batteries seems to depend on the testing conditions. Due to the cross increase of polysulfide substances, positive electrode sulfur precipitation was indeed observed at high temperatures, and further work is needed to improve the performance of Fe/S systems and compete with other flow batteries.

